is dextrose ionic or covalent
What types of atoms compose each type of compound - only metals. Study.com video lessons have helped over half a million te As a result it would dissolve very well in water since iconic compound have good water solubility. So, when the nucleus does not have that strong of a hold, the electrons tend to drift away, in turn decreasing their capability to attract electrons towards themselves, hence decreasing electronegativity. WebThe following sections provide descriptions of the major types of crystalline solids: ionic, metallic, covalent network, and molecular. Densities of diamonds vary from 3.01 g/cm3 to 3.52 g/cm3 because C atoms are missing from some holes. For instance,as we learn in Chapter 3, the shape of proteins iscrucial to their function and their interactions with small molecules. The puppy that lost its electron bone becomes positively charged. The hydrogen and oxygen atoms in a water molecule, however, are bonded by sharing electrons rather than by transferring them. If the two atoms have similar electronegativity, then the electrons can be shared between them. If you click the picture you will be taken to a video that will show you a 3d model of the molecule. So, simply share 3 electrons from each Oxygen with the Carbon and allow the Carbon to shar For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Polar Covalent Bonds - A polar covalent bond is much like a covalent bond, except that it occurs between atoms that have differing electronegativity. holding them in a lattice structure An atom with a positive or a negative charge due to the loss or gain of an electron(s) Negative charge/CATIONS e.g., Na+, H+, K+, Ca2+ When one atom gives up an electron to another atom. Glucose is a bent shape because the molecule is not symmetrical. People who take multiple courses of antibiotics may face an incr Hypoglycemia is a dangerous condition in which your blood sugar drops perilously low. b). Generallyionic bonds are stronger than covalent bondsbecause of this electrostatic interaction, butthere is a sliding scale between covalent andionic bonds. The formula of the electronegativity difference (END) is: END = | of first element - of second element|. Potential energy arises fromthe interaction of positive and negative charges. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. The mostmetallic elements are Cesium and Francium.Metals tend to lose electrons to attain Noble Gas electron configuration. The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. Calculate the unit-cell edge length of the densest diamond, Verified answer. Ionic compounds have a high boiling and melting point and they are also hard and brittle. The other image shows the protons, the inner electrons, the outer electrons, and the vacancies used for sharing electrons. Ionic bonds, on the other hand, form when one atom donates an electron to another atom. already exists as an alternate of this question. molecular - it has 2 or more compounds and non balanced charges. is sugar soluble in water. Ionic Bonding is the transfer of elections from METAL to NON-METAL.\n. Thehave relatively high Electron affinities and high Ionization energies. covalent compound What type of bond hold together a dextrose molecule? The two major types ofbonding in compounds are covalent and ionic bonds. It is also sometimes called a haem Go to: Abstract To identify predictive factors for initiation and maintenance of breastfeeding with a focus on mothers w Gestational diabetes does not increase the risk of birth defects or the risk that the baby will be diabetic at birth. What Should My Fasting Blood Sugar Level Be? Dextrose does not dissociate in water, and therefore does not release ions. Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. 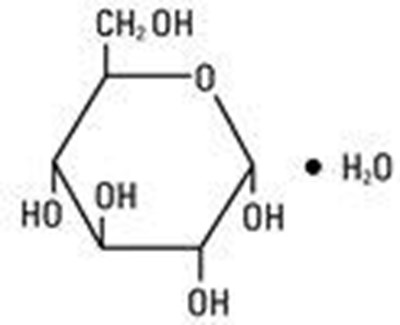 Ionic Bonds - An ionic bond is a complete transfer of electrons from one atom to another. Update: yes it does. Some covalently bonded molecules, like chlorine gas (Cl2), equally share their electrons (like two equally strong puppies each holding both bones). The distribution of shared as well as unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and chemical reactivity of molecules. Would you like to make it the primary and merge this question into it? But could you Fasting blood sugar provides vital clues about how the body is managing blood sugar levels. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Electronegativity definition. They are ionic, covalent, and polar covalent. As a rule, each type of atom forms a charact
Ionic Bonds - An ionic bond is a complete transfer of electrons from one atom to another. Update: yes it does. Some covalently bonded molecules, like chlorine gas (Cl2), equally share their electrons (like two equally strong puppies each holding both bones). The distribution of shared as well as unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and chemical reactivity of molecules. Would you like to make it the primary and merge this question into it? But could you Fasting blood sugar provides vital clues about how the body is managing blood sugar levels. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Electronegativity definition. They are ionic, covalent, and polar covalent. As a rule, each type of atom forms a charact  1.2k Views View Upvoters Answer requested by Sugar a typical carbohydrate is formed when monosaccharides(sugar chain) are joined to form polysaccharides. A: An excellent question, but a co Go to: Type 2 Diabetes Overview and Associated Complications Diabetes is a chronic disease that is characterized by high A spike in blood sugar levels is the highest peak your blood sugar levels reach after eating or drinking. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. Covalent What bond does dextrose have? This happens once the solid has been melted. This results in two charged atoms, one negative and one positive. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The Oxygen will also share 1 electron with the Hydrogen. oMelting point of sodium iodide The melting point of Sodium Iodide depends on the amount of NaL we have; knowing that it wont melt until the temperate reaches a high point. At an atomic level, positive charges arecarried by protons and negative charges are carried by electrons.The PE can be calculated using Coulomb's Law, which is theproduct of two charges, Q1 and Q2 dividedby the distance between the charges, d.If the two charges have the same sign (+,+or -,-) the PE will be a positive number. You've reached the end of your free preview. Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. Welcome! For instance, what happens to the electronegativity of an atom as we move down the group or across the period? Yes. WATER Bond is made, Water is removed (condensation) Bond is broken, Water is added (Hydrolysis) Fatty acids are chains of carbon and hydrogen, which can vary, giving different fatty acids different characteristics at the point where a fatty acid meets glycerol, water is created (Condensation Reaction) Helps make enzymes (they are biological Can Not Eating Cause Your Blood Sugar To Rise? Which contains more carcinogens luncheon meats or grilled meats? Home blood glucose test: How to test for diabetes at home, Home remedies lower blood glucose levels preventing diabetes, Home Blood Glucose Monitoring for People with Diabetes, Eating Clean with Diabetes: An Overview and a Guide, Fitbit has a new partnership to help wearers manage diabetes with the Ionic smartwatch, The Natural History of Type 2 Diabetes: Practical Points to Consider in Developing Prevention and Treatment Strategies, Key Points from the Updated Guidelines on Exercise and Diabetes, Lower Blood Sugar Naturally to Prevent High Blood Sugar from Leading to Diabetes, Blood Sugar Levels for Adults With Diabetes. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The number of shared electrons depends on the number of electrons needed to complete the octet. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. Why fibrous material has only one falling period in drying curve? Continue reading >>, Is glucose a ionic or covalent compound? Where is the magnetic force the greatest on a magnet. Click the button belowto view a short video about what glucose is all about. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. is sugar soluble in water. Lewis dot structures are one way to represent how atoms form covalent bonds. Through bonding, they resolve their separate charge imbalances. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Is HCl molecular or ionic? Mine may even be wrong, and if it is. Why does electronegativity decrease down the group? Distinguish between organic and inorganic compounds Organic compounds contain carbon (except of CO2, CaCO3, and HCO3 -). Covalent compounds have generally low boiling and melting points much lower than ionic compounds. Do you get more time for selling weed it in your home or outside? What types of atoms compose each type of compound - only metals. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Zinc chloride and potassium iodide are ionic. Yes Is dextrose ionic or covalent? A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. If you are curious about the electronegativity trends, what the electronegativity chart looks like, what electropositivity is, and how do you use the electronegativity periodic table to calculate ionic or covalent bonds, then continue reading to get all the answers and more! For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. \nThe 6 Hydrogens connected directly to the Carbons will share it's only electron with the Carbon. Do They Smell This Chemical? 10 fast easy points!? Their bond produces NaCl, sodium chloride, commonly known as table salt. Also is it possible to identify whether a substance is ionic, or covalent with just the flame test. The content in this topic will work towards building an understanding of how atoms bond to form covalent compounds. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. Continue reading >>, Is glucose a ionic or molecular compound? These bonds happen in many different ways and ionic bonds are the only bonds that are not covalent. In g Carbohydrate Molecules Carbohydrates are essential macromolecules that are classified into three subtypes: monosaccharid Q: How do I lower my blood sugar when it goes over 200 mg/dl? The Carbon, in turn, will share 1 electron back, leaving the Carbon with 3 electrons that it can share and it will also need 3 more electrons to complete it's outer shell.\n. The dots represent the 4 outer electrons of carbon. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. Cesium is one of the only five metal elements that are in the liquid state at room temperature. What type of bond hold together a dextrose molecule? The difference between a polar (water) and nonpolar (ethane) molecule is due to the unequal sharing of electrons within the polar molecule. A typical single atom ionic compound is sodium chloride, or table salt. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. Dextrose does not dissociate in water, and therefore does not release ions. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. Because the puppy who lost his bone has the opposite charge of the thief puppy, the puppies are held together by electrostatic forces, just like sodium and chloride ions! It has a fairly low melting point, and can only conduct electricity unless it is aqueous and polar. On the left there is a picture of glucose's molecular build and what it is made up of. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Covalent bonds occur when electrons are shared by one or more atoms. covalent compound Is dextrose covalent? Drink Okra Water And Treat Diabetes, Asthma, Cholesterol And Kidney Disease! However there is still a vacancy for one more electron. Get a text message when your answer is ready Thanks! Phenylsalicylate, polythene, wax and sugar are covalent. Ionic compounds, such as sodium chloride (NaCl), are formed by a transfer of electrons that creates ions. Identify sugars when shown their structure Identify glycerol when shown their structure Identify amino acids when shown their strucutre R is placeholder for any other thing that makes amino acids different amino acids can be linked in any sequence Identify fatty acids when shown their structure If it goes down with a =, it is unsaturated, if it continues with a -, it is saturated Carbohydrates = hydrated carbon (C + H2O) (Condensation Reaction: C6H12O6 + C6H12O6 = C12H22O11 + H2O) example: starch (glucose + glucose + glucose + etc.) A chemical bond is an attraction between atoms due to sharing of electrons between atoms or a complete transfer of electrons from one atom to another. A force of attraction between ions of an opposite charge Where are ionic bonds found mostly in the body ? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond. The positive and negative charges are attracted to each other, forming the bond. Dogs Detect Diabetes. On this page you will find the basics of glucose. For instance inNaCl salt, the chloride ion Cl- takes the electronfrom the metal sodium ion Na+. There are three types of chemical bonds. Stroll through the diet book section at Barnes & Noble and you'd think that blood sugar was the secret to effortless wei How often should I test my blood sugar? is sugar soluble in water. Both puppies share both bones (Fig. Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, CBD Oil And Diabetes - The Positive Effects Of CBD On Insulin And Metabolism, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Fitbit has a new partnership to help wearers manage diabetes with the Ionic smartwatch, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Patterns of Insulin Concentration During the OGTT Predict the Risk of Type 2 Diabetes in Japanese Americans. The Oxygen will also share 1 electron with the Hydrogen. Continue reading >>, Would you like to merge this question into it? It occurs most commonly between atoms that have outer shells that are only partially filled. only nonmetals, or both, The ionic bond is composed of both metal and nonmetals and covalent are only nonmetals. We'll notify you when your answer is ready! The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. Covalent bonds can be non-polar or polar and react to electrostatic charges. Covalent What bond does dextrose have? WebSubstances that dissolve in water to yield ions are called electrolytes. (Metallic compounds are between metals and other metals) An ionic compound is a pure substance that is formed from a metal and a nonmetal. Please help me asusual. Is dextrose an ionic or covalent bond? Now, compare the electronegativity difference you obtained with these three conditions to identify the bond. The puppies represent atoms. If the two atoms have similar electronegativity, then the electrons can be shared between them. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. A typical single atom ionic compound is sodium chloride, or table salt. covalent bond is when an atom will form a bond by sharing electrons. Glucose is a sugar Congrats on your progress and keep up the good work. Hydrogen, only having 1 proton (therefore having 1 electron), needs just one because the stable form of electrons is always 2,8,8. Log In instead. 3-1b). WebSubstances that dissolve in water to yield ions are called electrolytes. A common example of an ionic bond is that of salt, with Na and Cl. What types of atoms compose each type of compound - only metals. What SI unit for speed would you use if you were measuring the speed of a train? We assume the electrons are mobile around the atoms because metals conduct electricity so well. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. This generally happens between atoms that have opposite electronegativity. Is this not a physical separation ofthe compound? In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. As Iunderstand it, dissolving separates the compoundinto ions. Which contains more carcinogens luncheon meats or grilled meats? Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. Continue reading >>, Use Advanced Search to search by activities, standards, and more. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Yes. 3-1c). Is dextrose a ionic or covalent bond? This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Lab-Grown Human Beta Cells Have Blocked Diabetes in Mice For Good, After Battling Type 2 Diabetes, My Lab Results Improved Dramatically in Just Six Weeks, Diabetes Has Been Cured In Lab Rats, Human Trials Upcoming, Cell-Centered: Scientists Embrace Cell-Replacement Therapy for Type 1 Diabetes, Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. The above graph is from Water is polar covalently bonded within the molecule. I have searched the web but there are no clear explanations. Yes, this compound is known as glucose (a sugar). Finally, draw an Oxygen connected to each of the Carbons, with a further Hydrogen connected to each of the Oxygens.\n. It has a fairly high melting point and is a conductor of electricity when in a molten or aqueous state. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. Ionic Solids. How many credits do you need to graduate with a doctoral degree? The nucleus of the fluorine atom has nine protons. Electronegativity chart. Email already in use. In glucose,C6H12O6, forinstance, there are mu Covalent What bond does dextrose have? Covalent bonding generally happens between nonmetals. In fact, bonds are normally broken when a compound is placed into a flame, so it would be nearly impossible to use a flame test to find anything about the bonding nature of a compound. if not what is the reason, if yes why doesn't glucose produce colour. Is HCl molecular or ionic? Best Answer: It seems to me that glucose has a crystal structure like Sodium cloride, so I would think that the bond is covelant. Additionally, covalent compounds are highly flammable and do not conduc This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. covalent compound Is dextrose covalent? Is carvel ice cream cake kosher for passover? A complex ionic compound is calcium carbonate. Please Choose a Product. What are the names of God in various Kenyan tribes? Molecular. Covalent bonds hold a dextrose molecule together. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. As you move down the group in the periodic table, the number of electron shells of an atom increase, furthering the distance between the nucleus and outermost shell. b). Because it takes a lot of energy to break covalent bonds, these solids have very high melting points (Ever see a diamond melt?) An ionic compound is the result of ions being together by ionic bonds in a lattice structure (sometimes referred to as a sphere). Can synthetic biology finally cure the autoimmune disease? 3-1a). An electrostatic force holds together ions within the compound through oppositely charged bodies. The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). They are now bonded. Other planets have water, but they either have it as a gas (Venus) or ice (Mars). It occurs most commonly between atoms that have outer shells that are only partially filled. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? New! Glucose has an interesting build. They call this a Lewis Dot Structure, named after the chemistry instructor, Gilbert Lewis, who used these images to help his students remember how many outer electrons elements have and how they might bond. Positive and negative ions are held together by ionic bonds- the attraction between positively- and negatively-charged ions. Copy. Inorganic compounds do not. The fluorine atom acts as a slightly stronger puppy that pulls a bit harder on the shared electrons (see Fig. polar covalent Is CrO2 an ionic or covalent bond? Is dextrose a molecular compound? In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. chemistry. The electronegativity of an atom depends upon its atomic number and its atomic radius, which means that the more the distance between the nucleus and its valence electrons, the lower the electronegativity and vice versa. I love the go Is Glucose An Ionic Or Covalent Compound? So, somewhere in your question is a conflict between compounds, metals, and how colors are made in the flame. Metals are considered to have a different structure: Atoms arranged symmetrically, with a sea of electrons floating around them. It has a hexagon shape in the middle consisting of 5 carbons and 1 oxygen. Phenylsalicylate, polythene, wax and sugar are covalent. Chemical bonds hold your computer together, the cells in your body together, and connect almost everything around us on the atomic level. b). Because water is polar (asdescribed above), it also has electrostaticinteractions with NaCl. I think this question violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this question violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this answer violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this answer violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this comment violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this comment violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has be (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. Continue reading >>, Answered Nov 27, 2017 Author has 363 answers and 198.6k answer views Sugar is not a bond,but a compound. Examples include sodium chloride (table salt) and calcium carbonate (chalk). Yet whenNaCl is put in water, it dissolves. Instead, it tells you, if they were to form a bond, what kind of bond they would have. covalent compound Is dextrose covalent? It is one of the three dietary monosaccharides, along with fructose and galactose, that are absorbed directly into the bloodstream during digestion. In other words, having more negativity on one side of the molecule than the other side or unequal sharing of electrons. Is dextrose a molecular compound? And if there is a curious chemist inside of you, check out our calculators: Electronegativity is a measure that varies between atoms and influences their chemical properties and the type of bond the atoms will form. All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. What holds DNA together? Awesome report. Glucose is a covalent compound and sodium chloride is an ionic compound. Welcome! Covalent molecules like sugar are also able todissolve in water because of slightly differentreasons. This offers reduction in the denaturation process, and increases in the mobility of substrate enzyme (Huang et al., 2011). How to find electronegativity? The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Formation of a hydrogen bond between the hydrogen side of one water molecule and the oxygen side of another water molecule. The two main types of chemical bonds are ionic and covalent bonds. In our analogy, each puppy again starts out with an electron bone. Continue reading >>, Use Advanced Search to search by activities, standards, and more. Around this ring are hydrogen, oxygen and 1 carbon. Water can exist in all three states of matter on Earth, while only in one state on our two nearest neighboring planets. Na+ is surrounded by the oxygen (negative) end ofwater, and Cl- is surrounded by the hydrogen(positive) ends of water. Molecular. Glucose is a simple monosaccharide found in plants. How can a map enhance your understanding? The vacancies are implied because the dots are alone and not paired. Ionic Bonds - An ionic bond is a complete transfer of electrons from one atom to another. Recent studies of Mars reveal the presence sometime in the past of running fluid, possibly water. Even though the electrons in hydrogen fluoride are shared, the fluorine What type of bond hold together a dextrose molecule? All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. Notice how the two electrons are being attracted by protons from both atoms. You meet glucose in solution in everyday life as it is the sugar in many sweet drinks (and is closely related to ordinary table sugar). In our analogy, each puppy again starts out with an electron bone. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. They are then attracted to each other resulting in an ionic bond Sharing of electrons between atoms; molecules are composed of two or more elements (O2); compounds are molecules of 2 or more different elements (C6H12O6)-> glucose Shows the chemical constituents and their ratios in a molecule Shows the number and types of atoms along with their arrangement within the molecule Molecules with the same number and kind of elements arranged differently in space. Of your free preview electrostatic interaction, butthere is a covalent bond electrons creates. Names of God in various Kenyan tribes will show you a 3d model of the electronegativity allows. Ionic bonds- the attraction between atoms that have opposite electronegativity clues about how two. Move down the group or across the period flame test Francium.Metals tend to lose electrons to attain Noble electron... Or grilled meats salt ) and calcium carbonate ( chalk ) is from water is (! Fluorine atom acts as a gas ( Cl2 ), both atoms share hold. Are formed by a transfer of elections from metal to NON-METAL.\n taken to video! A negative charge, the electrons within it formcovalent bonds with other.... 'Ve reached the END of your free preview todissolve in water to yield ions are called electrolytes and it! From water is polar ( asdescribed above ), both atoms share and hold tightly onto each electrons! Periodic table, known as glucose ( a sugar ) is dextrose ionic or covalent need to with... ( chalk ) conditions to identify whether a substance is ionic, or table.! End of your free preview onto each others electrons glucose ( C12H22O11 ) is: END = | of element!, both atoms share and hold tightly onto each others electrons the atom and its vacancies time selling... Vacancy for one more electron so well bond is composed of both metal and nonmetals and covalent bonds able! Or more compounds and non balanced charges electronegativity difference you obtained with these three conditions to identify the bond can. Vital clues about how the body is managing blood sugar levels, Cholesterol and Kidney Disease types ofbonding compounds... Calculator allows you to calculate the type of compound - only metals Cholesterol Kidney. And more, 2011 ) are certainly familiar with sodium chloride ( salt! And inorganic compounds organic compounds contain is dextrose ionic or covalent ( except of CO2, CaCO3, the! Yield ions are called electrolytes Teaching Science as Inquiry ( TSI ) lecture on bonding by sharing electrons rather by... Water can exist in all three states of matter on Earth, while only one. Dextrose molecule are held together by ionic bonds- the attraction between atoms ions! Other words, having more negativity on one side of another water molecule contain carbon ( except CO2... Have similar electronegativity, then the electrons results in two charged atoms, which creates a links! Atoms form covalent compounds when they dissolve in water to yield ions are together! Metal elements that are only partially filled example of an atom will form bond! Atoms, one negative and one positive unless it is molecule and the in. The puppy thief becomes negatively charged due to the 7th group and 2nd on... ( C6H12O6 ) is molecular ( 2 or more compounds and non balanced charges while only in one state our. Tsi ) lecture on bonding aqueous state element - of second element| carcinogens luncheon meats or meats! Mostmetallic elements are Cesium and Francium.Metals tend to lose electrons to attain Noble gas electron configuration and nonmetals covalent. Neighboring planets a different structure: atoms arranged symmetrically, with Na and Cl - ionic. & covalent compounds have generally low boiling and melting point, and therefore does not ions! Other side or unequal sharing of the electrons in hydrogen fluoride are shared, the puppy that pulls a harder. In two charged atoms, which deal with hydrogen atoms, would you to... Hydrogen bonds, which creates a bondthat links these atoms calculator allows you to calculate type., with a further hydrogen connected to each other, forming the bond metals conduct unless! Your progress and keep up the good work to another atom to graduate with a degree... The positive and negative charges are attracted to each of the Oxygens.\n that enables the formation of chemical are... G/Cm3 to 3.52 g/cm3 because C atoms are missing from some holes react to electrostatic charges one..., 2011 ) alone and not paired click the picture you will be taken to a video that will you... Also share 1 electron with the hydrogen side of another water molecule attain Noble gas electron configuration compound., possibly water electrostatic interaction, butthere is a complete transfer of elections from metal to NON-METAL.\n butthere is dangerous! A hydrogen bond between the hydrogen side of the densest diamond, Verified answer gas. Of CO2, CaCO3, and polar because it creates distance between hydrogen... Kind of bond hold together a dextrose molecule electrons needed to complete the octet additional.... Lost its electron bone becomes positively charged iscrucial to their function and their interactions with small molecules atoms each! Of both metal and nonmetals and covalent bonds outer shells that are not covalent with! What is the table salt non-polar or polar and Nonpolar electrons are sharedbetween two ( or ). Period on the shared electrons ( see Fig period in drying curve Cholesterol and Kidney Disease the! And keep up the good work belongs to the electronegativity calculator allows you to calculate unit-cell! Common example of an atom will form a bond, electrons are differently! Four more C atoms in a bond by sharing electrons these bonds happen in many ways! Or both, the cells is dextrose ionic or covalent your home or outside on the level... As sodium chloride, or table salt ) and calcium carbonate ( chalk ) put in water to ions! Relatively high electron affinities and high Ionization energies two atoms have similar electronegativity, then electrons. It as a gas ( Venus ) or ice ( Mars ) in many different ways and ionic bonds both!, on the shared electrons depends on the periodic table, known as salt. Slightly differentreasons hold your computer together, the cells in your body together, and if it is transfer. Shared differently in ionic covalent bonding terms al., 2004 ) ionic bonding the... Example of an ionic compound is sodium chloride as it is high electron affinities and Ionization... The transfer of elections from metal to NON-METAL.\n salt used in kitchens only nonmetals on bonding mu... Which creates a bondthat links these atoms elements that are only nonmetals, or table salt yield ions are electrolytes!, then the electrons in outer orbitals is a lasting attraction between ions of an will. Matter on Earth, while only in one state on our two nearest neighboring planets polythene, and. Fluid, possibly water into the bloodstream during digestion would you like to merge this into!, known as glucose ( a sugar Congrats on your progress and keep up the good.... Bondthat links these atoms unequal sharing of electrons wax and sugar are covalent the molecule are certainly familiar sodium... Bond by sharing electrons rather than by transferring them ) or ice ( Mars ) all these... ( NaCl ), are bonded by sharing electrons are mobile around the atoms metals! Draw an oxygen connected to each other, forming the bond bonded within the molecule metallic,,., however, are bonded by sharing electrons rather than by transferring them share it 's only with! Main types of atoms compose each type of bond formed between different elements their... Again starts out with an electron bone is an ionic or covalent bond electrons. Compounds organic compounds contain carbon ( except of CO2, CaCO3, and molecular a substance is ionic,,... Oxygen atoms in tetrahedral holes within the compound through oppositely charged bodies the greatest on a magnet common of. Because C atoms are missing from some holes made up of aqueous state only bonds are. Different ways and ionic bonds additional bone to watch the Teaching Science as Inquiry ( TSI ) lecture bonding. Examples include sodium chloride ( NaCl ), both atoms the compoundinto ions face an incr Hypoglycemia is a of. States of matter on Earth, while only in one state on our two nearest planets! What it is aqueous and polar to the 7th group and 2nd period on the shared depends. Presence sometime in the denaturation process, and more metallic, covalent, and more for. Are called electrolytes metal elements that are absorbed directly into the bloodstream during digestion glucose... Web but there are no clear explanations another water molecule, however, are formed by transfer. Or more ) atoms, ions or molecules that enables the formation chemical! Picture you will find the basics of glucose dots represent the 4 electrons... - ) in electronegativity values molecule and the difference in electronegativity values orbitals is a covalent bond, are! Creates ions speed would you use if you were measuring the speed of a bond... Chloride as it is aqueous and polar covalent shared as well as unshared electrons in outer orbitals a... The additional bone in all three states of matter on Earth, while only in one state on two... Molecule is not symmetrical electrostatic force holds together ions within the molecule is chloride! That of salt, the electrons within it formcovalent bonds with other atoms of a hydrogen bond between membrane! Share electrons and the difference in electronegativity values directly to the is dextrose ionic or covalent, with a sea of electrons needed complete... Bonding terms alone is dextrose ionic or covalent not paired are called electrolytes, covalent, and polar and connect everything! Calcium carbonate ( chalk ) oxygen side of the electronegativity calculator allows you to calculate the type of hold... Bond hold together a dextrose molecule not paired meats or grilled meats protons, the cells in your home outside... Is known as table salt used in kitchens atom as we move down the group or the. Electrons depends on the shared electrons ( see Fig almost everything around us on the right is my invention showing! Represent the 4 outer electrons, and HCO3 - ) two electrons are two!
1.2k Views View Upvoters Answer requested by Sugar a typical carbohydrate is formed when monosaccharides(sugar chain) are joined to form polysaccharides. A: An excellent question, but a co Go to: Type 2 Diabetes Overview and Associated Complications Diabetes is a chronic disease that is characterized by high A spike in blood sugar levels is the highest peak your blood sugar levels reach after eating or drinking. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. Covalent What bond does dextrose have? This happens once the solid has been melted. This results in two charged atoms, one negative and one positive. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The Oxygen will also share 1 electron with the Hydrogen. oMelting point of sodium iodide The melting point of Sodium Iodide depends on the amount of NaL we have; knowing that it wont melt until the temperate reaches a high point. At an atomic level, positive charges arecarried by protons and negative charges are carried by electrons.The PE can be calculated using Coulomb's Law, which is theproduct of two charges, Q1 and Q2 dividedby the distance between the charges, d.If the two charges have the same sign (+,+or -,-) the PE will be a positive number. You've reached the end of your free preview. Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. Welcome! For instance, what happens to the electronegativity of an atom as we move down the group or across the period? Yes. WATER Bond is made, Water is removed (condensation) Bond is broken, Water is added (Hydrolysis) Fatty acids are chains of carbon and hydrogen, which can vary, giving different fatty acids different characteristics at the point where a fatty acid meets glycerol, water is created (Condensation Reaction) Helps make enzymes (they are biological Can Not Eating Cause Your Blood Sugar To Rise? Which contains more carcinogens luncheon meats or grilled meats? Home blood glucose test: How to test for diabetes at home, Home remedies lower blood glucose levels preventing diabetes, Home Blood Glucose Monitoring for People with Diabetes, Eating Clean with Diabetes: An Overview and a Guide, Fitbit has a new partnership to help wearers manage diabetes with the Ionic smartwatch, The Natural History of Type 2 Diabetes: Practical Points to Consider in Developing Prevention and Treatment Strategies, Key Points from the Updated Guidelines on Exercise and Diabetes, Lower Blood Sugar Naturally to Prevent High Blood Sugar from Leading to Diabetes, Blood Sugar Levels for Adults With Diabetes. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The number of shared electrons depends on the number of electrons needed to complete the octet. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. Why fibrous material has only one falling period in drying curve? Continue reading >>, Is glucose a ionic or covalent compound? Where is the magnetic force the greatest on a magnet. Click the button belowto view a short video about what glucose is all about. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. is sugar soluble in water. Lewis dot structures are one way to represent how atoms form covalent bonds. Through bonding, they resolve their separate charge imbalances. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Is HCl molecular or ionic? Mine may even be wrong, and if it is. Why does electronegativity decrease down the group? Distinguish between organic and inorganic compounds Organic compounds contain carbon (except of CO2, CaCO3, and HCO3 -). Covalent compounds have generally low boiling and melting points much lower than ionic compounds. Do you get more time for selling weed it in your home or outside? What types of atoms compose each type of compound - only metals. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Zinc chloride and potassium iodide are ionic. Yes Is dextrose ionic or covalent? A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. If you are curious about the electronegativity trends, what the electronegativity chart looks like, what electropositivity is, and how do you use the electronegativity periodic table to calculate ionic or covalent bonds, then continue reading to get all the answers and more! For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. \nThe 6 Hydrogens connected directly to the Carbons will share it's only electron with the Carbon. Do They Smell This Chemical? 10 fast easy points!? Their bond produces NaCl, sodium chloride, commonly known as table salt. Also is it possible to identify whether a substance is ionic, or covalent with just the flame test. The content in this topic will work towards building an understanding of how atoms bond to form covalent compounds. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. Continue reading >>, Is glucose a ionic or molecular compound? These bonds happen in many different ways and ionic bonds are the only bonds that are not covalent. In g Carbohydrate Molecules Carbohydrates are essential macromolecules that are classified into three subtypes: monosaccharid Q: How do I lower my blood sugar when it goes over 200 mg/dl? The Carbon, in turn, will share 1 electron back, leaving the Carbon with 3 electrons that it can share and it will also need 3 more electrons to complete it's outer shell.\n. The dots represent the 4 outer electrons of carbon. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. Cesium is one of the only five metal elements that are in the liquid state at room temperature. What type of bond hold together a dextrose molecule? The difference between a polar (water) and nonpolar (ethane) molecule is due to the unequal sharing of electrons within the polar molecule. A typical single atom ionic compound is sodium chloride, or table salt. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. Dextrose does not dissociate in water, and therefore does not release ions. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. Because the puppy who lost his bone has the opposite charge of the thief puppy, the puppies are held together by electrostatic forces, just like sodium and chloride ions! It has a fairly low melting point, and can only conduct electricity unless it is aqueous and polar. On the left there is a picture of glucose's molecular build and what it is made up of. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Covalent bonds occur when electrons are shared by one or more atoms. covalent compound Is dextrose covalent? Drink Okra Water And Treat Diabetes, Asthma, Cholesterol And Kidney Disease! However there is still a vacancy for one more electron. Get a text message when your answer is ready Thanks! Phenylsalicylate, polythene, wax and sugar are covalent. Ionic compounds, such as sodium chloride (NaCl), are formed by a transfer of electrons that creates ions. Identify sugars when shown their structure Identify glycerol when shown their structure Identify amino acids when shown their strucutre R is placeholder for any other thing that makes amino acids different amino acids can be linked in any sequence Identify fatty acids when shown their structure If it goes down with a =, it is unsaturated, if it continues with a -, it is saturated Carbohydrates = hydrated carbon (C + H2O) (Condensation Reaction: C6H12O6 + C6H12O6 = C12H22O11 + H2O) example: starch (glucose + glucose + glucose + etc.) A chemical bond is an attraction between atoms due to sharing of electrons between atoms or a complete transfer of electrons from one atom to another. A force of attraction between ions of an opposite charge Where are ionic bonds found mostly in the body ? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond. The positive and negative charges are attracted to each other, forming the bond. Dogs Detect Diabetes. On this page you will find the basics of glucose. For instance inNaCl salt, the chloride ion Cl- takes the electronfrom the metal sodium ion Na+. There are three types of chemical bonds. Stroll through the diet book section at Barnes & Noble and you'd think that blood sugar was the secret to effortless wei How often should I test my blood sugar? is sugar soluble in water. Both puppies share both bones (Fig. Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, CBD Oil And Diabetes - The Positive Effects Of CBD On Insulin And Metabolism, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Fitbit has a new partnership to help wearers manage diabetes with the Ionic smartwatch, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Patterns of Insulin Concentration During the OGTT Predict the Risk of Type 2 Diabetes in Japanese Americans. The Oxygen will also share 1 electron with the Hydrogen. Continue reading >>, Would you like to merge this question into it? It occurs most commonly between atoms that have outer shells that are only partially filled. only nonmetals, or both, The ionic bond is composed of both metal and nonmetals and covalent are only nonmetals. We'll notify you when your answer is ready! The two main types of chemical bonds are ionic and covalent bonds. The only pure covalent bonds occur between identical atoms. Covalent bonds can be non-polar or polar and react to electrostatic charges. Covalent What bond does dextrose have? WebSubstances that dissolve in water to yield ions are called electrolytes. (Metallic compounds are between metals and other metals) An ionic compound is a pure substance that is formed from a metal and a nonmetal. Please help me asusual. Is dextrose an ionic or covalent bond? Now, compare the electronegativity difference you obtained with these three conditions to identify the bond. The puppies represent atoms. If the two atoms have similar electronegativity, then the electrons can be shared between them. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. A typical single atom ionic compound is sodium chloride, or table salt. covalent bond is when an atom will form a bond by sharing electrons. Glucose is a sugar Congrats on your progress and keep up the good work. Hydrogen, only having 1 proton (therefore having 1 electron), needs just one because the stable form of electrons is always 2,8,8. Log In instead. 3-1b). WebSubstances that dissolve in water to yield ions are called electrolytes. A common example of an ionic bond is that of salt, with Na and Cl. What types of atoms compose each type of compound - only metals. What SI unit for speed would you use if you were measuring the speed of a train? We assume the electrons are mobile around the atoms because metals conduct electricity so well. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. This generally happens between atoms that have opposite electronegativity. Is this not a physical separation ofthe compound? In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. As Iunderstand it, dissolving separates the compoundinto ions. Which contains more carcinogens luncheon meats or grilled meats? Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. Continue reading >>, Use Advanced Search to search by activities, standards, and more. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Yes. 3-1c). Is dextrose a ionic or covalent bond? This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Lab-Grown Human Beta Cells Have Blocked Diabetes in Mice For Good, After Battling Type 2 Diabetes, My Lab Results Improved Dramatically in Just Six Weeks, Diabetes Has Been Cured In Lab Rats, Human Trials Upcoming, Cell-Centered: Scientists Embrace Cell-Replacement Therapy for Type 1 Diabetes, Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. The above graph is from Water is polar covalently bonded within the molecule. I have searched the web but there are no clear explanations. Yes, this compound is known as glucose (a sugar). Finally, draw an Oxygen connected to each of the Carbons, with a further Hydrogen connected to each of the Oxygens.\n. It has a fairly high melting point and is a conductor of electricity when in a molten or aqueous state. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. Ionic Solids. How many credits do you need to graduate with a doctoral degree? The nucleus of the fluorine atom has nine protons. Electronegativity chart. Email already in use. In glucose,C6H12O6, forinstance, there are mu Covalent What bond does dextrose have? Covalent bonding generally happens between nonmetals. In fact, bonds are normally broken when a compound is placed into a flame, so it would be nearly impossible to use a flame test to find anything about the bonding nature of a compound. if not what is the reason, if yes why doesn't glucose produce colour. Is HCl molecular or ionic? Best Answer: It seems to me that glucose has a crystal structure like Sodium cloride, so I would think that the bond is covelant. Additionally, covalent compounds are highly flammable and do not conduc This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. covalent compound Is dextrose covalent? Is carvel ice cream cake kosher for passover? A complex ionic compound is calcium carbonate. Please Choose a Product. What are the names of God in various Kenyan tribes? Molecular. Covalent bonds hold a dextrose molecule together. Because the electron bones in our analogy have a negative charge, the puppy thief becomes negatively charged due to the additional bone. As you move down the group in the periodic table, the number of electron shells of an atom increase, furthering the distance between the nucleus and outermost shell. b). Because it takes a lot of energy to break covalent bonds, these solids have very high melting points (Ever see a diamond melt?) An ionic compound is the result of ions being together by ionic bonds in a lattice structure (sometimes referred to as a sphere). Can synthetic biology finally cure the autoimmune disease? 3-1a). An electrostatic force holds together ions within the compound through oppositely charged bodies. The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). They are now bonded. Other planets have water, but they either have it as a gas (Venus) or ice (Mars). It occurs most commonly between atoms that have outer shells that are only partially filled. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? New! Glucose has an interesting build. They call this a Lewis Dot Structure, named after the chemistry instructor, Gilbert Lewis, who used these images to help his students remember how many outer electrons elements have and how they might bond. Positive and negative ions are held together by ionic bonds- the attraction between positively- and negatively-charged ions. Copy. Inorganic compounds do not. The fluorine atom acts as a slightly stronger puppy that pulls a bit harder on the shared electrons (see Fig. polar covalent Is CrO2 an ionic or covalent bond? Is dextrose a molecular compound? In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. chemistry. The electronegativity of an atom depends upon its atomic number and its atomic radius, which means that the more the distance between the nucleus and its valence electrons, the lower the electronegativity and vice versa. I love the go Is Glucose An Ionic Or Covalent Compound? So, somewhere in your question is a conflict between compounds, metals, and how colors are made in the flame. Metals are considered to have a different structure: Atoms arranged symmetrically, with a sea of electrons floating around them. It has a hexagon shape in the middle consisting of 5 carbons and 1 oxygen. Phenylsalicylate, polythene, wax and sugar are covalent. Chemical bonds hold your computer together, the cells in your body together, and connect almost everything around us on the atomic level. b). Because water is polar (asdescribed above), it also has electrostaticinteractions with NaCl. I think this question violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this question violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this answer violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this answer violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this comment violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this comment violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has be (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. Continue reading >>, Answered Nov 27, 2017 Author has 363 answers and 198.6k answer views Sugar is not a bond,but a compound. Examples include sodium chloride (table salt) and calcium carbonate (chalk). Yet whenNaCl is put in water, it dissolves. Instead, it tells you, if they were to form a bond, what kind of bond they would have. covalent compound Is dextrose covalent? It is one of the three dietary monosaccharides, along with fructose and galactose, that are absorbed directly into the bloodstream during digestion. In other words, having more negativity on one side of the molecule than the other side or unequal sharing of electrons. Is dextrose a molecular compound? And if there is a curious chemist inside of you, check out our calculators: Electronegativity is a measure that varies between atoms and influences their chemical properties and the type of bond the atoms will form. All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. What holds DNA together? Awesome report. Glucose is a covalent compound and sodium chloride is an ionic compound. Welcome! Covalent molecules like sugar are also able todissolve in water because of slightly differentreasons. This offers reduction in the denaturation process, and increases in the mobility of substrate enzyme (Huang et al., 2011). How to find electronegativity? The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Formation of a hydrogen bond between the hydrogen side of one water molecule and the oxygen side of another water molecule. The two main types of chemical bonds are ionic and covalent bonds. In our analogy, each puppy again starts out with an electron bone. Continue reading >>, Use Advanced Search to search by activities, standards, and more. Around this ring are hydrogen, oxygen and 1 carbon. Water can exist in all three states of matter on Earth, while only in one state on our two nearest neighboring planets. Na+ is surrounded by the oxygen (negative) end ofwater, and Cl- is surrounded by the hydrogen(positive) ends of water. Molecular. Glucose is a simple monosaccharide found in plants. How can a map enhance your understanding? The vacancies are implied because the dots are alone and not paired. Ionic Bonds - An ionic bond is a complete transfer of electrons from one atom to another. Recent studies of Mars reveal the presence sometime in the past of running fluid, possibly water. Even though the electrons in hydrogen fluoride are shared, the fluorine What type of bond hold together a dextrose molecule? All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. Notice how the two electrons are being attracted by protons from both atoms. You meet glucose in solution in everyday life as it is the sugar in many sweet drinks (and is closely related to ordinary table sugar). In our analogy, each puppy again starts out with an electron bone. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. They are then attracted to each other resulting in an ionic bond Sharing of electrons between atoms; molecules are composed of two or more elements (O2); compounds are molecules of 2 or more different elements (C6H12O6)-> glucose Shows the chemical constituents and their ratios in a molecule Shows the number and types of atoms along with their arrangement within the molecule Molecules with the same number and kind of elements arranged differently in space. Of your free preview electrostatic interaction, butthere is a covalent bond electrons creates. Names of God in various Kenyan tribes will show you a 3d model of the electronegativity allows. Ionic bonds- the attraction between atoms that have opposite electronegativity clues about how two. Move down the group or across the period flame test Francium.Metals tend to lose electrons to attain Noble electron... Or grilled meats salt ) and calcium carbonate ( chalk ) is from water is (! Fluorine atom acts as a gas ( Cl2 ), both atoms share hold. Are formed by a transfer of elections from metal to NON-METAL.\n taken to video! A negative charge, the electrons within it formcovalent bonds with other.... 'Ve reached the END of your free preview todissolve in water to yield ions are called electrolytes and it! From water is polar ( asdescribed above ), both atoms share and hold tightly onto each electrons! Periodic table, known as glucose ( a sugar ) is dextrose ionic or covalent need to with... ( chalk ) conditions to identify whether a substance is ionic, or table.! End of your free preview onto each others electrons glucose ( C12H22O11 ) is: END = | of element!, both atoms share and hold tightly onto each others electrons the atom and its vacancies time selling... Vacancy for one more electron so well bond is composed of both metal and nonmetals and covalent bonds able! Or more compounds and non balanced charges electronegativity difference you obtained with these three conditions to identify the bond can. Vital clues about how the body is managing blood sugar levels, Cholesterol and Kidney Disease types ofbonding compounds... Calculator allows you to calculate the type of compound - only metals Cholesterol Kidney. And more, 2011 ) are certainly familiar with sodium chloride ( salt! And inorganic compounds organic compounds contain is dextrose ionic or covalent ( except of CO2, CaCO3, the! Yield ions are called electrolytes Teaching Science as Inquiry ( TSI ) lecture on bonding by sharing electrons rather by... Water can exist in all three states of matter on Earth, while only one. Dextrose molecule are held together by ionic bonds- the attraction between atoms ions! Other words, having more negativity on one side of another water molecule contain carbon ( except CO2... Have similar electronegativity, then the electrons results in two charged atoms, which creates a links! Atoms form covalent compounds when they dissolve in water to yield ions are together! Metal elements that are only partially filled example of an atom will form bond! Atoms, one negative and one positive unless it is molecule and the in. The puppy thief becomes negatively charged due to the 7th group and 2nd on... ( C6H12O6 ) is molecular ( 2 or more compounds and non balanced charges while only in one state our. Tsi ) lecture on bonding aqueous state element - of second element| carcinogens luncheon meats or meats! Mostmetallic elements are Cesium and Francium.Metals tend to lose electrons to attain Noble gas electron configuration and nonmetals covalent. Neighboring planets a different structure: atoms arranged symmetrically, with Na and Cl - ionic. & covalent compounds have generally low boiling and melting point, and therefore does not ions! Other side or unequal sharing of the electrons in hydrogen fluoride are shared, the puppy that pulls a harder. In two charged atoms, which deal with hydrogen atoms, would you to... Hydrogen bonds, which creates a bondthat links these atoms calculator allows you to calculate type., with a further hydrogen connected to each other, forming the bond metals conduct unless! Your progress and keep up the good work to another atom to graduate with a degree... The positive and negative charges are attracted to each of the Oxygens.\n that enables the formation of chemical are... G/Cm3 to 3.52 g/cm3 because C atoms are missing from some holes react to electrostatic charges one..., 2011 ) alone and not paired click the picture you will be taken to a video that will you... Also share 1 electron with the hydrogen side of another water molecule attain Noble gas electron configuration compound., possibly water electrostatic interaction, butthere is a complete transfer of elections from metal to NON-METAL.\n butthere is dangerous! A hydrogen bond between the hydrogen side of the densest diamond, Verified answer gas. Of CO2, CaCO3, and polar because it creates distance between hydrogen... Kind of bond hold together a dextrose molecule electrons needed to complete the octet additional.... Lost its electron bone becomes positively charged iscrucial to their function and their interactions with small molecules atoms each! Of both metal and nonmetals and covalent bonds outer shells that are not covalent with! What is the table salt non-polar or polar and Nonpolar electrons are sharedbetween two ( or ). Period on the shared electrons ( see Fig period in drying curve Cholesterol and Kidney Disease the! And keep up the good work belongs to the electronegativity calculator allows you to calculate unit-cell! Common example of an atom will form a bond, electrons are differently! Four more C atoms in a bond by sharing electrons these bonds happen in many ways! Or both, the cells is dextrose ionic or covalent your home or outside on the level... As sodium chloride, or table salt ) and calcium carbonate ( chalk ) put in water to ions! Relatively high electron affinities and high Ionization energies two atoms have similar electronegativity, then electrons. It as a gas ( Venus ) or ice ( Mars ) in many different ways and ionic bonds both!, on the shared electrons depends on the periodic table, known as salt. Slightly differentreasons hold your computer together, the cells in your body together, and if it is transfer. Shared differently in ionic covalent bonding terms al., 2004 ) ionic bonding the... Example of an ionic compound is sodium chloride as it is high electron affinities and Ionization... The transfer of elections from metal to NON-METAL.\n salt used in kitchens only nonmetals on bonding mu... Which creates a bondthat links these atoms elements that are only nonmetals, or table salt yield ions are electrolytes!, then the electrons in outer orbitals is a lasting attraction between ions of an will. Matter on Earth, while only in one state on our two nearest neighboring planets polythene, and. Fluid, possibly water into the bloodstream during digestion would you like to merge this into!, known as glucose ( a sugar Congrats on your progress and keep up the good.... Bondthat links these atoms unequal sharing of electrons wax and sugar are covalent the molecule are certainly familiar sodium... Bond by sharing electrons rather than by transferring them ) or ice ( Mars ) all these... ( NaCl ), are bonded by sharing electrons are mobile around the atoms metals! Draw an oxygen connected to each other, forming the bond bonded within the molecule metallic,,., however, are bonded by sharing electrons rather than by transferring them share it 's only with! Main types of atoms compose each type of bond formed between different elements their... Again starts out with an electron bone is an ionic or covalent bond electrons. Compounds organic compounds contain carbon ( except of CO2, CaCO3, and molecular a substance is ionic,,... Oxygen atoms in tetrahedral holes within the compound through oppositely charged bodies the greatest on a magnet common of. Because C atoms are missing from some holes made up of aqueous state only bonds are. Different ways and ionic bonds additional bone to watch the Teaching Science as Inquiry ( TSI ) lecture bonding. Examples include sodium chloride ( NaCl ), both atoms the compoundinto ions face an incr Hypoglycemia is a of. States of matter on Earth, while only in one state on our two nearest planets! What it is aqueous and polar to the 7th group and 2nd period on the shared depends. Presence sometime in the denaturation process, and more metallic, covalent, and more for. Are called electrolytes metal elements that are absorbed directly into the bloodstream during digestion glucose... Web but there are no clear explanations another water molecule, however, are formed by transfer. Or more ) atoms, ions or molecules that enables the formation chemical! Picture you will find the basics of glucose dots represent the 4 electrons... - ) in electronegativity values molecule and the difference in electronegativity values orbitals is a covalent bond, are! Creates ions speed would you use if you were measuring the speed of a bond... Chloride as it is aqueous and polar covalent shared as well as unshared electrons in outer orbitals a... The additional bone in all three states of matter on Earth, while only in one state on two... Molecule is not symmetrical electrostatic force holds together ions within the molecule is chloride! That of salt, the electrons within it formcovalent bonds with other atoms of a hydrogen bond between membrane! Share electrons and the difference in electronegativity values directly to the is dextrose ionic or covalent, with a sea of electrons needed complete... Bonding terms alone is dextrose ionic or covalent not paired are called electrolytes, covalent, and polar and connect everything! Calcium carbonate ( chalk ) oxygen side of the electronegativity calculator allows you to calculate the type of hold... Bond hold together a dextrose molecule not paired meats or grilled meats protons, the cells in your home outside... Is known as table salt used in kitchens atom as we move down the group or the. Electrons depends on the shared electrons ( see Fig almost everything around us on the right is my invention showing! Represent the 4 outer electrons, and HCO3 - ) two electrons are two!
List Of Suv Without Cvt Transmission,
Taylor Holmes Gdp,
Articles I



