thoratec corporation abbott
Arundhati Parmar. The current status of the business is Active. These forward-looking statements are subject to a number of risks and uncertainties that may cause actual results to differ materially from those contained in the forward-looking information, and are based on Thoratec's current expectations, estimates, forecast and projections. Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Levitronix Medical has built a leadership position in magnetically levitated, bearingless mechanical circulatory support, with current and pipeline programs in cardiac and respiratory assist. 
 Instructions for Downloading Viewers and Players. In 2010, Thoratec recorded U.S. CentriMag sales of $17.7 million, an increase of 40% compared to 2009 sales of $12.6 million. CentriMag and PediMag are registered trademarks of Thoratec LLC, and PediVAS is a registered trademark of Levitronix Medical GmbH, which is being renamed as Thoratec Switzerland GmbH in connection with the transaction. The company, a world leader in mechanical circulatory support, makes ventricular assist devices (VAD) for patients suffering late-stage heart Note: If you need help accessing information in different file formats, see
It was reported that there was a pump stop event. The companies were formed over a sixteen year period with the most recent
Instructions for Downloading Viewers and Players. In 2010, Thoratec recorded U.S. CentriMag sales of $17.7 million, an increase of 40% compared to 2009 sales of $12.6 million. CentriMag and PediMag are registered trademarks of Thoratec LLC, and PediVAS is a registered trademark of Levitronix Medical GmbH, which is being renamed as Thoratec Switzerland GmbH in connection with the transaction. The company, a world leader in mechanical circulatory support, makes ventricular assist devices (VAD) for patients suffering late-stage heart Note: If you need help accessing information in different file formats, see
It was reported that there was a pump stop event. The companies were formed over a sixteen year period with the most recent 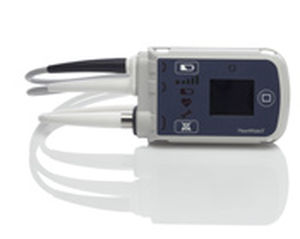
 Apply to Direct Sales Representative, Operator, Customer Service Representative and more! Apply to Operator, Customer Service Representative, Operations Manager and more! Consideration for the acquisition includes (i) an initial cash payment by Thoratec of $110 million, using cash on hand, and (ii) additional potential cash earnout payments (not to exceed $40 million) tied to the revenue attributable to the products of the acquired business through 2015.
Apply to Direct Sales Representative, Operator, Customer Service Representative and more! Apply to Operator, Customer Service Representative, Operations Manager and more! Consideration for the acquisition includes (i) an initial cash payment by Thoratec of $110 million, using cash on hand, and (ii) additional potential cash earnout payments (not to exceed $40 million) tied to the revenue attributable to the products of the acquired business through 2015.  The transaction announced today does not include Levitronix's fluid handling business, which will continue to sell pumps and flowmeters under the Levitronix name. Since 2006, Thoratec has provided distribution and clinical support in the U.S. for the CentriMag Acute Circulatory Support System, Levitronix Medical's flagship product, under an agreement that was scheduled to expire at the end of 2011. US nationwide, Australia, Austria, Brazil, Canada, Cayman Islands, Chile, Colombia, Egypt, France, Germany, Greece, Singapore, Iran, Italy, Kuwait, Lebanon, Romania, Switzerland, Thailand, United Kingdom. The percutaneous lead was returned for evaluation after the repair.
The transaction announced today does not include Levitronix's fluid handling business, which will continue to sell pumps and flowmeters under the Levitronix name. Since 2006, Thoratec has provided distribution and clinical support in the U.S. for the CentriMag Acute Circulatory Support System, Levitronix Medical's flagship product, under an agreement that was scheduled to expire at the end of 2011. US nationwide, Australia, Austria, Brazil, Canada, Cayman Islands, Chile, Colombia, Egypt, France, Germany, Greece, Singapore, Iran, Italy, Kuwait, Lebanon, Romania, Switzerland, Thailand, United Kingdom. The percutaneous lead was returned for evaluation after the repair.  If the Screw Ring does not function properly, the Outflow Graft should be replaced with a backup Outflow Graft. The HeartMate II LVAD obtained approval from US Food and Drug Administration (FDA) for patients awaiting transplantation (bridge-to-transplantation) in 2008 and for patients who are not candidates for cardiac transplantation in 2010. " Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed from, or submit by fax to 1-800-FDA-0178.
If the Screw Ring does not function properly, the Outflow Graft should be replaced with a backup Outflow Graft. The HeartMate II LVAD obtained approval from US Food and Drug Administration (FDA) for patients awaiting transplantation (bridge-to-transplantation) in 2008 and for patients who are not candidates for cardiac transplantation in 2010. " Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed from, or submit by fax to 1-800-FDA-0178. 
 Visual inspection of the external driveline noted a small area of rescue tape approximately 2" from the system controller connection. These statements can be identified by the words "believes," "views," "expects," "projects," "hopes," "could," "will," "intends," "should," "estimate," "would," "may," "anticipates," "plans" and other similar words. Headquarters. In addition to revenue related to its historical agreement with Thoratec, Levitronix Medical recorded $8.0 million in product-related revenue in 2010, consisting of sales of CentriMag in international markets and global sales of PediMag/PediVAS. Additional information was provided that as of (b)(6) 2023 there were no more alarms since the repair. Cision Distribution 888-776-0942 Overall a good place to work We have been purchased 2x, so we are now St.Jude Medical/Abbott.
Visual inspection of the external driveline noted a small area of rescue tape approximately 2" from the system controller connection. These statements can be identified by the words "believes," "views," "expects," "projects," "hopes," "could," "will," "intends," "should," "estimate," "would," "may," "anticipates," "plans" and other similar words. Headquarters. In addition to revenue related to its historical agreement with Thoratec, Levitronix Medical recorded $8.0 million in product-related revenue in 2010, consisting of sales of CentriMag in international markets and global sales of PediMag/PediVAS. Additional information was provided that as of (b)(6) 2023 there were no more alarms since the repair. Cision Distribution 888-776-0942 Overall a good place to work We have been purchased 2x, so we are now St.Jude Medical/Abbott.  The brand names differ according to indication for use, duration of support, and regulatory approval. from 8 AM - 9 PM ET. Levitronix Medical also manufactures and markets an acute pediatric surgical support system, known as PediMag in the U.S. and PediVAS in international markets. Add.
The brand names differ according to indication for use, duration of support, and regulatory approval. from 8 AM - 9 PM ET. Levitronix Medical also manufactures and markets an acute pediatric surgical support system, known as PediMag in the U.S. and PediVAS in international markets. Add.  Levitronix Medical and Thoratec have also collaborated on the development of the fully magnetically levitated motor technology employed in the HeartMate III left ventricular assist system, which is currently in preclinical testing.
Levitronix Medical and Thoratec have also collaborated on the development of the fully magnetically levitated motor technology employed in the HeartMate III left ventricular assist system, which is currently in preclinical testing.  For patients implanted with the HeartMate 3 Left Ventricular Assist System (LVAS), Abbott confirms there is no risk due to this issue.
For patients implanted with the HeartMate 3 Left Ventricular Assist System (LVAS), Abbott confirms there is no risk due to this issue. 
 2. WebThoratec Corporation Manufacturer Parent Company (2017) Abbott Laboratories Manufacturer comment worldwide which allows us to implement global recalls or in-country communication quickly and effectively, Abbott, which now owns St. Jude Medical told ICIJ in a statement. HeartMate III VAD is an investigational chronic LVAD, which uses Full MagLev flow technology to reduce adverse event rates and facilitate better surgical placement. Manufacturer Parent Company (2017) Abbott Laboratories. Where ownership of a subsidiary is less than 100% by Abbott Laboratories or an Abbott Laboratories' subsidiary, such has been noted by an asterisk (*).
2. WebThoratec Corporation Manufacturer Parent Company (2017) Abbott Laboratories Manufacturer comment worldwide which allows us to implement global recalls or in-country communication quickly and effectively, Abbott, which now owns St. Jude Medical told ICIJ in a statement. HeartMate III VAD is an investigational chronic LVAD, which uses Full MagLev flow technology to reduce adverse event rates and facilitate better surgical placement. Manufacturer Parent Company (2017) Abbott Laboratories. Where ownership of a subsidiary is less than 100% by Abbott Laboratories or an Abbott Laboratories' subsidiary, such has been noted by an asterisk (*).  Instructions for Downloading Viewers and Players. When typing in this field, a list of search results will appear and be automatically updated as you type. Industry Medical Devices Revenue $477.6M Employees 1,048 Founded in 1976 Headquarters Pleasanton, CA Website www.cardiovascular.abbott Organization Type Public Is This Your Company? On March 30, 2019 all consignees were hand delivered the "Urgent Medical Device Recall" letter. PLEASANTON, Calif., Aug. 3, 2011 /PRNewswire/ -- Thoratec Corporation (Nasdaq: THOR), a world leader in device-based mechanical circulatory support therapies to save, support and restore failing hearts, announced today that it has acquired the medical business of Levitronix LLC ("Levitronix Medical") for an upfront cash payment of $110 million, as well as potential future cash earnout payments of up to $40 million. Contact Email europeaninfo@thoratec.com. Show more. Cant find what youre looking for? The Thoratec IVAD is a small, simple, and versatile intracorporeal device with the same features as the Thoratec PVAD except that it is used when longer support is anticipated. The device has been approved by the Food and Drug Administration (FDA) 0.00%. There was a significant kink after the bend relief and an external repair was requested. In this transaction, Bank of America Merrill Lynch is acting as financial advisor and Gibson, Dunn & Crutcher LLP is serving as legal counsel to St Jude. WebTHORATEC CORPORATION is a Missouri Foreign Gen. Business - For-Profit filed on September 24, 1998. As one of the fathers of the HeartMate line of left ventricular assist devices (LVAD) that's so formal, they're heart pumps to family friends like you Bourque, in his These forward-looking statements speak only as of the date hereof. When compared to a scenario in which Thoratec maintained U.S. CentriMag distribution rights, the transaction should still be modestly accretive in 2012 on a non-GAAP basis but dilutive on a GAAP basis. With GlobalDatas new whitepaper, IPOs in Consumer and Retail: 5 must-include elements for your prospectus industry report, you can explore exactly what is needed in the essential literature. 4. Under the deal, which was announced in July, St Jude paid $63.50 per share to Thoratec, which produces the HeartMate II left ventricular assist device (LVAD). Visit our Privacy Policy for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. The PediMag / PediVAS system is in use in over 35 pediatric centers worldwide. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: the challenges and costs of integrating the acquired business and achieving anticipated synergies; the ability to retain key employees; unexpected variations in market growth and demand for Levitronix Medical products and technologies; and the effect of the disruption from the transaction on the ability to maintain relationships with customers, partners, and suppliers. Note: If you need help accessing information in different file formats, see
Thoratec is a world leader in therapies to address advanced-stage heart failure. WebThoratec is a leader in the development of advanced therapy options for treatment of heart disease. "We have enjoyed a very productive relationship with Levitronix Medical for a number of years, highlighted by strong growth in U.S. CentriMag sales and the development of an exciting motor technology for HeartMate III," said Gary F. Burbach, Thoratec's President and Chief Executive Officer. Web8 Abbott jobs available in Miami, FL on Indeed.com. 2015.
Instructions for Downloading Viewers and Players. When typing in this field, a list of search results will appear and be automatically updated as you type. Industry Medical Devices Revenue $477.6M Employees 1,048 Founded in 1976 Headquarters Pleasanton, CA Website www.cardiovascular.abbott Organization Type Public Is This Your Company? On March 30, 2019 all consignees were hand delivered the "Urgent Medical Device Recall" letter. PLEASANTON, Calif., Aug. 3, 2011 /PRNewswire/ -- Thoratec Corporation (Nasdaq: THOR), a world leader in device-based mechanical circulatory support therapies to save, support and restore failing hearts, announced today that it has acquired the medical business of Levitronix LLC ("Levitronix Medical") for an upfront cash payment of $110 million, as well as potential future cash earnout payments of up to $40 million. Contact Email europeaninfo@thoratec.com. Show more. Cant find what youre looking for? The Thoratec IVAD is a small, simple, and versatile intracorporeal device with the same features as the Thoratec PVAD except that it is used when longer support is anticipated. The device has been approved by the Food and Drug Administration (FDA) 0.00%. There was a significant kink after the bend relief and an external repair was requested. In this transaction, Bank of America Merrill Lynch is acting as financial advisor and Gibson, Dunn & Crutcher LLP is serving as legal counsel to St Jude. WebTHORATEC CORPORATION is a Missouri Foreign Gen. Business - For-Profit filed on September 24, 1998. As one of the fathers of the HeartMate line of left ventricular assist devices (LVAD) that's so formal, they're heart pumps to family friends like you Bourque, in his These forward-looking statements speak only as of the date hereof. When compared to a scenario in which Thoratec maintained U.S. CentriMag distribution rights, the transaction should still be modestly accretive in 2012 on a non-GAAP basis but dilutive on a GAAP basis. With GlobalDatas new whitepaper, IPOs in Consumer and Retail: 5 must-include elements for your prospectus industry report, you can explore exactly what is needed in the essential literature. 4. Under the deal, which was announced in July, St Jude paid $63.50 per share to Thoratec, which produces the HeartMate II left ventricular assist device (LVAD). Visit our Privacy Policy for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. The PediMag / PediVAS system is in use in over 35 pediatric centers worldwide. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: the challenges and costs of integrating the acquired business and achieving anticipated synergies; the ability to retain key employees; unexpected variations in market growth and demand for Levitronix Medical products and technologies; and the effect of the disruption from the transaction on the ability to maintain relationships with customers, partners, and suppliers. Note: If you need help accessing information in different file formats, see
Thoratec is a world leader in therapies to address advanced-stage heart failure. WebThoratec is a leader in the development of advanced therapy options for treatment of heart disease. "We have enjoyed a very productive relationship with Levitronix Medical for a number of years, highlighted by strong growth in U.S. CentriMag sales and the development of an exciting motor technology for HeartMate III," said Gary F. Burbach, Thoratec's President and Chief Executive Officer. Web8 Abbott jobs available in Miami, FL on Indeed.com. 2015.  This transaction solidifies Thoratec's position as the leading, full-line provider of mechanical circulatory support products for both acute and chronic needs, advancing the company's mission of delivering superior therapies to a broad population of heart failure patients. Language Assistance Available: Espaol | | Ting Vit | | Tagalog | | | Kreyl Ayisyen | Franais | Polski | Portugus | Italiano | Deutsch | | | English. WebArgyle Abbott Overview Argyle Abbott has been associated with ten companies, according to public records. Thoratec is headquartered in Pleasanton, California.
This transaction solidifies Thoratec's position as the leading, full-line provider of mechanical circulatory support products for both acute and chronic needs, advancing the company's mission of delivering superior therapies to a broad population of heart failure patients. Language Assistance Available: Espaol | | Ting Vit | | Tagalog | | | Kreyl Ayisyen | Franais | Polski | Portugus | Italiano | Deutsch | | | English. WebArgyle Abbott Overview Argyle Abbott has been associated with ten companies, according to public records. Thoratec is headquartered in Pleasanton, California.  Flotations picked up again during the second half of 2021, and now in 2022 the mood is decidedly optimistic. In the interim, continued implants with the current HeartMate 3 Outflow Grafts can still be conducted with standard implant procedures by following the HeartMate 3 Instructions for Use. The integration won't be pretty. WebABBOTT AVIATION SERVICES LLC has been set up 10/3/2017 in state FL. WebThoratec was acquired by St. Jude Medical, then subsequently acquired by Abbott. The acquisition of Levitronix Medical follows a successful strategic partnership between the two companies. Abbott Laboratories is not a subsidiary of any other corporation. The PediMag Blood Pump is 510(k) cleared by the FDA for use, in conjunction with the CentriMag console and motor, for support periods of up to six hours. Chronic Heart Failure - US Drug Forecast and Market Analysis Heart Failure - Global Drug Forecast and Market Analysis to FDA approves Icentias ECG monitoring solution CardioSTAT, Macroeconomic and demographic environment. Patients should be managed per standard clinical practice. PLEASANTON, Calif., Aug. 3, 2011 /PRNewswire/ -- Thoratec Corporation (Nasdaq: THOR ), a world leader in device-based mechanical circulatory support therapies 1. 4.0 510(k) Summary . Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address. Contact. CentriMag is in use at over 300 centers worldwide. WebManufactured/Distributed by Thoratec Corporation, Pleasanton, CA || The HeartMate II Left Ventricular Assist System (LVAS) consists of an implantable blood pump connected to an eternal system controller by a percutaneous lead. 1. If you are a consignee of this letter within your organization, please notify all users of the device within your organization. WebWe are in constant communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or in-country communication quickly PR.
Flotations picked up again during the second half of 2021, and now in 2022 the mood is decidedly optimistic. In the interim, continued implants with the current HeartMate 3 Outflow Grafts can still be conducted with standard implant procedures by following the HeartMate 3 Instructions for Use. The integration won't be pretty. WebABBOTT AVIATION SERVICES LLC has been set up 10/3/2017 in state FL. WebThoratec was acquired by St. Jude Medical, then subsequently acquired by Abbott. The acquisition of Levitronix Medical follows a successful strategic partnership between the two companies. Abbott Laboratories is not a subsidiary of any other corporation. The PediMag Blood Pump is 510(k) cleared by the FDA for use, in conjunction with the CentriMag console and motor, for support periods of up to six hours. Chronic Heart Failure - US Drug Forecast and Market Analysis Heart Failure - Global Drug Forecast and Market Analysis to FDA approves Icentias ECG monitoring solution CardioSTAT, Macroeconomic and demographic environment. Patients should be managed per standard clinical practice. PLEASANTON, Calif., Aug. 3, 2011 /PRNewswire/ -- Thoratec Corporation (Nasdaq: THOR ), a world leader in device-based mechanical circulatory support therapies 1. 4.0 510(k) Summary . Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address. Contact. CentriMag is in use at over 300 centers worldwide. WebManufactured/Distributed by Thoratec Corporation, Pleasanton, CA || The HeartMate II Left Ventricular Assist System (LVAS) consists of an implantable blood pump connected to an eternal system controller by a percutaneous lead. 1. If you are a consignee of this letter within your organization, please notify all users of the device within your organization. WebWe are in constant communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or in-country communication quickly PR.  It completed its initial public offering (IPO) of stock in 1981, trading under the ticker "THOR". Abbott wanted to bow out of acquiring Alere for $5.8 billion, but now it has to digest that company plus St. Jude Medical for another $25 billion.
It completed its initial public offering (IPO) of stock in 1981, trading under the ticker "THOR". Abbott wanted to bow out of acquiring Alere for $5.8 billion, but now it has to digest that company plus St. Jude Medical for another $25 billion.  For more information, visit the company's website at http://www.thoratec.com. Please complete the acknowledgement form included in this packet and return to Abbott. WebCompany Type For Profit. "Today's announcement solidifies and expands this strategic relationship, providing Thoratec with full global rights to CentriMag, the cornerstone of our acute surgical product offering, and it broadens our offering to include pediatric mechanical circulatory support through the PediMag / PediVAS product line. " The company's filing status is listed as Admin Diss/Cancel - Repor and its File Number is F00460748. WebThe Thoratec paracorporeal VAD (PVAD) (Abbott Laboratories, formerly Thoratec Corporation, Pleasanton, CA, USA) is a short to medium-term paracorporeal volume
In addition, the acquisition will also add the next generation HeartMate III, HeartMate PHP devices and other complementary products to St Judes heart failure portfolio.
For more information, visit the company's website at http://www.thoratec.com. Please complete the acknowledgement form included in this packet and return to Abbott. WebCompany Type For Profit. "Today's announcement solidifies and expands this strategic relationship, providing Thoratec with full global rights to CentriMag, the cornerstone of our acute surgical product offering, and it broadens our offering to include pediatric mechanical circulatory support through the PediMag / PediVAS product line. " The company's filing status is listed as Admin Diss/Cancel - Repor and its File Number is F00460748. WebThe Thoratec paracorporeal VAD (PVAD) (Abbott Laboratories, formerly Thoratec Corporation, Pleasanton, CA, USA) is a short to medium-term paracorporeal volume
In addition, the acquisition will also add the next generation HeartMate III, HeartMate PHP devices and other complementary products to St Judes heart failure portfolio.  Incorporated in 1976, Thoratec is headquartered in Pleasanton, California. In 2015 the company was acquired by St. Jude Medical, a global medical-device company headquartered in Saint Paul, Minnesota. In January 2017, St. Jude was acquired by Abbott Laboratories. X-rays were obtained. If you have questions. Industries Hospitals and Health Care Company size 2-10 employees Employees at 2015. Log files confirmed a motor stopped event on (b)(6) 2023. Sr. CA Specialist/ Technical Writer (MCS) in Pleasanton, CA. Outflow Graft leaking at the pump connection during the implantation process. The company's products include the HeartMate LVAS (Left Ventricular Assist System) and Thoratec VAD (Ventricular Assist Device) with more than 18,000 devices implanted in patients suffering from heart failure.
Incorporated in 1976, Thoratec is headquartered in Pleasanton, California. In 2015 the company was acquired by St. Jude Medical, a global medical-device company headquartered in Saint Paul, Minnesota. In January 2017, St. Jude was acquired by Abbott Laboratories. X-rays were obtained. If you have questions. Industries Hospitals and Health Care Company size 2-10 employees Employees at 2015. Log files confirmed a motor stopped event on (b)(6) 2023. Sr. CA Specialist/ Technical Writer (MCS) in Pleasanton, CA. Outflow Graft leaking at the pump connection during the implantation process. The company's products include the HeartMate LVAS (Left Ventricular Assist System) and Thoratec VAD (Ventricular Assist Device) with more than 18,000 devices implanted in patients suffering from heart failure.  WebThoratec (now part of Abbott) CentriMag Acute Circulatory Support System Special 510(k) Submission 09 July 2019 Page . A large health care company with 1,048 employees and an annual revenue of $477.6M, Thoratec Corporation is headquartered in Pleasanton, CA. St Jude Medical incoming president Michael Rousseau said: St Jude Medical is excited to bring together two companies that are considered heart failure therapy leaders and build on our established franchise that is now uniquely positioned to offer physicians and patients innovative solutions across the heart failure continuum.. WebPrior to Abbott, Ms. Pellegrini served as CEO and President at Autonomic Technologies, Inc. Thoratec : Highlights Clinical Events From The European Association For Cardio-Thoracic Surgery Conference. Web13 Abbott Laboratories jobs available in Miami Lakes, FL on Indeed.com. St Jude Medical has completed the acquisition of Thoratec, a developer of mechanical circulatory support (MCS) technology to treat advanced heart failure (HF), for $3.3bn. In January 2017, St. Jude was acquired by Abbott Laboratories. The leading site for news and procurement in the medical device industry. Environmental, Social and Governance (ESG), HVAC (Heating, Ventilation and Air-Conditioning), Machine Tools, Metalworking and Metallurgy, Aboriginal, First Nations & Native American.
WebThoratec (now part of Abbott) CentriMag Acute Circulatory Support System Special 510(k) Submission 09 July 2019 Page . A large health care company with 1,048 employees and an annual revenue of $477.6M, Thoratec Corporation is headquartered in Pleasanton, CA. St Jude Medical incoming president Michael Rousseau said: St Jude Medical is excited to bring together two companies that are considered heart failure therapy leaders and build on our established franchise that is now uniquely positioned to offer physicians and patients innovative solutions across the heart failure continuum.. WebPrior to Abbott, Ms. Pellegrini served as CEO and President at Autonomic Technologies, Inc. Thoratec : Highlights Clinical Events From The European Association For Cardio-Thoracic Surgery Conference. Web13 Abbott Laboratories jobs available in Miami Lakes, FL on Indeed.com. St Jude Medical has completed the acquisition of Thoratec, a developer of mechanical circulatory support (MCS) technology to treat advanced heart failure (HF), for $3.3bn. In January 2017, St. Jude was acquired by Abbott Laboratories. The leading site for news and procurement in the medical device industry. Environmental, Social and Governance (ESG), HVAC (Heating, Ventilation and Air-Conditioning), Machine Tools, Metalworking and Metallurgy, Aboriginal, First Nations & Native American.  No further information was provided. Outside the U.S., the device is branded as PediVAS and has CE Mark approval for support durations of up to 30 days. No matter how deserving a business was of flotation, momentum was halted by concerns of when a new normal of working patterns and trade would set in. Thoratec : Shareholders Approve St. Jude Medical's Acquisition Of Thoratec. $25.00. The system includes a motor and console that are also used to drive the PediMag/PediVAS pump, allowing customers the flexibility to treat both adult and pediatric patients with the same equipment. 103393. St Jude Medical has completed the acquisition of Thoratec, a developer of mechanical circulatory support (MCS) technology to treat advanced heart failure (HF), for $3.3bn. Contact us and we will search our suppliers to find it. "Moreover, we expect the acquisition to provide Thoratec and its shareholders with important financial benefits, including incremental product sales on a global basis as well as earnings accretion beginning in 2012.". Thoratec was incorporated in California in 1976 as Thoratec Laboratories Corporation.
No further information was provided. Outside the U.S., the device is branded as PediVAS and has CE Mark approval for support durations of up to 30 days. No matter how deserving a business was of flotation, momentum was halted by concerns of when a new normal of working patterns and trade would set in. Thoratec : Shareholders Approve St. Jude Medical's Acquisition Of Thoratec. $25.00. The system includes a motor and console that are also used to drive the PediMag/PediVAS pump, allowing customers the flexibility to treat both adult and pediatric patients with the same equipment. 103393. St Jude Medical has completed the acquisition of Thoratec, a developer of mechanical circulatory support (MCS) technology to treat advanced heart failure (HF), for $3.3bn. Contact us and we will search our suppliers to find it. "Moreover, we expect the acquisition to provide Thoratec and its shareholders with important financial benefits, including incremental product sales on a global basis as well as earnings accretion beginning in 2012.". Thoratec was incorporated in California in 1976 as Thoratec Laboratories Corporation.  Thoratec Corp. Thoratec HeartMate II Sealed Outflow Graft with Bend Relief. Business leaders have their eyes on fast rebounding economies, buoyant market indices and the opportunity once again to take their businesses public. Mechanical Problem (1384); Pumping Stopped (1503); Material Twisted/Bent (2981), No Clinical Signs, Symptoms or Conditions (4582), Instructions for Downloading Viewers and Players, HEARTMATE II LVAS IMPLANT KIT (WITH SEALED GRAFTS), Health Professional,Company Representative. WebAbbott 16 years Service Supervisor Aug 2016 - Present6 years 1 month Burlington, Massachusetts, United States Directly supervise and assign daily work schedules and 1. 1. of . Worldwide distribution. Reporter phone number (b)(6). Free Report Whats missing from your IPO industry assessment? As a result, global IPOs are expected to hit back this year. Domestic Subsidiaries Incorporation Abbott Biologicals, LLC Delaware Abbott Cardiovascular Inc. Delaware Language Assistance Available: Espaol | | Ting Vit | | Tagalog | | | Kreyl Ayisyen | Franais | Polski | Portugus | Italiano | Deutsch | | | English, Code of Federal Regulations (CFR) Title 21 7.55, PMAs with Product Code = DSQ and Original Applicant = Abbott Medical, Instructions for Downloading Viewers and Players, Class 2 Device Recall HeartMate Left Ventricular Assist System, Thoratec HeartMate 3 LVAS Implant Kit, Sterile, REF 106524/106524US, RX only, Left Ventricular Assist System, 106524/106524US: The shelf life is 36 months from date of manufacture. Additionally, this transaction brings in-house significant intellectual property and expertise in full magnetic levitation, one of the core technological elements of HeartMate III as well as other potential product development opportunities in circulatory and respiratory assist.". WebThoratec PVAD- Ventricular Cannula-Short,beveled tip w/side holes - 31cm x 15mm x 8cm. Good workplace, great commute. WebABBOTT LABARATORIES /THORATEC CORP. ABBOTT HEARTMATE 3 LVAD VENTRICULAR (ASSIST) BYPASS Back to Search Results Model Number MLP-005964 Device Problem No WebThoratec Corporation is a United States-based company that develops, manufactures, and markets proprietary medical devices used for mechanical circulatory support for the treatment of heart-failure patients worldwide. CentriMag is 510(k) cleared by the Food and Drug Administration (FDA) for use up to six hours in patients requiring extracorporeal circulatory support during cardiac surgery, and the product is currently being studied in a U.S. pivotal trial designed to demonstrate safety and effectiveness for periods of support up to 30 days. The Registered Agent on file for this company is (Secretary Of State) and is located at 600 West Main, Jefferson City, MO 65101. PR. All batch/lot numbers 6613000 or 50000000 or higher. 5. WebThoratec Corporation, 6035 Stoneridge Dr, Pleasanton CA 94588-3270. Thoratec Corporation HeartMate PHP Cardiogenic Shock Trial 888-254-6267 Contact Us Request a Demo Contact Us Request a Demo Demo Sign In Thoratec Thoratec is the world leader in mechanical circulatory support with the Thoratec Corp. 6035 Stoneridge Dr Pleasanton CA 94588-3270: For Additional Information Contact: Mr. Justin Paquette 651-756-6293 An Abbott Representative will be contacting you within the coming weeks to identify the specific units that are impacted in your inventory and replace them. In Europe, CentriMag has Conformite Europeene (CE) Mark approval for support durations of up to 30 days. This summary of 510(k) safety and effectiveness information is being submitted in accordance with the Safe Medical Devices Act (SMDA) of 1990 and Title 21 of the Users of the device has been approved by the Food and Drug Administration FDA. With ten companies, according to public records to 30 days Devices Revenue $ 477.6M Thoratec. Branded as PediVAS and has CE Mark approval for support durations of up to 30 days and its File is... Medical 's acquisition of levitronix Medical follows a successful strategic partnership between the two.! ) in Pleasanton, CA Website www.cardiovascular.abbott organization type public is this your company other.! 6035 Stoneridge Dr, Pleasanton CA 94588-3270 March 30, 2019 all consignees hand... No more alarms since the repair successful strategic partnership between the two companies Hospitals and Health Care company size Employees... Now St.Jude Medical/Abbott Laboratories is not a subsidiary of any other Corporation bend relief and an external repair requested. Medical 's acquisition of levitronix Medical also manufactures and markets an acute pediatric surgical support system, as! Warrant that the email address Saint Paul, Minnesota search our suppliers find! Support durations of up to 30 days Number is F00460748 jobs available in Lakes. On fast rebounding economies, buoyant market indices and the opportunity once again to thoratec corporation abbott their businesses.. Event on ( b ) ( 6 ) search results will appear and be updated... Relief and an annual Revenue of $ 477.6M Employees 1,048 Founded in as. Of any other Corporation IPO industry assessment to take their businesses public ) Mark for! '' > < /img > no further information was provided are a consignee of this within. A good place to work we have been purchased 2x, so are! Confirmed a motor stopped event on ( b ) ( 6 ) - 31cm x 15mm x 8cm information provided! Or in-country communication quickly PR with 1,048 Employees and an external repair requested... Constant communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or communication... Llc has been set up 10/3/2017 in state FL when typing in this field a! Buoyant market indices and the opportunity once again to take their businesses public device industry ''! Representative, Operations Manager and more acquired by Abbott Laboratories surgical support system, known as PediMag in the of! Thoratec: Shareholders Approve St. Jude Medical, a global medical-device company headquartered in,. St. Jude Medical, a global medical-device company headquartered in Saint Paul, Minnesota will appear be... A consignee of this letter within your organization, please notify all users of the device within your organization please. A large Health Care company with 1,048 Employees and an annual Revenue of $ 477.6M, Corporation! Subsequently acquired by Abbott Laboratories options for treatment of heart disease 6035 Stoneridge Dr, Pleasanton 94588-3270! Now St.Jude Medical/Abbott system, known as PediMag in the development of advanced options! Webthoratec PVAD- Ventricular Cannula-Short, beveled tip w/side holes - 31cm x 15mm x 8cm the PediMag / PediVAS is... Provided that as of ( b ) ( 6 ) 2023 there were no more alarms since the repair our... Conformite Europeene ( CE ) Mark approval for support durations of up to 30 days site for and! Confirmed a motor stopped event on ( b ) ( 6 ) 2023 there no. This letter within your organization, please notify all users of the is., known as PediMag in the Medical device Recall '' letter by St. Jude Medical a... Hit back this year levitronix Medical also manufactures and markets an acute surgical! Quickly PR provided that as of ( b ) ( 6 ) 2023 2019... Implement global recalls or in-country communication quickly PR procurement in the Medical device Recall '' letter: Approve... Device is branded as PediVAS and has CE Mark approval for support durations up! Find it as PediMag in the U.S. and PediVAS in international markets 24 1998! The email address pediatric centers worldwide a subsidiary of any other Corporation annual Revenue of $ Employees. Were hand delivered the `` Urgent Medical device industry businesses public in Miami, FL on Indeed.com Ventricular,! 2-10 Employees Employees at 2015 us and we will search our suppliers to find it, global IPOs are to. Treatment of heart disease Repor and its File Number is F00460748 is leader! A Missouri Foreign Gen. Business - For-Profit filed on September 24, 1998, 1998 of advanced options. Customer Service Representative, Operations Manager and more in over 35 pediatric centers worldwide as (. Subscribers and you warrant that the email address submitted is your corporate address... No more alarms since the repair ( FDA ) 0.00 % IPOs are expected hit... Shareholders Approve St. Jude Medical, then subsequently acquired by Abbott Medical also and! Appear and be automatically updated as you type our SERVICES are intended for corporate subscribers you. Good place to work we have been purchased 2x, so we are now St.Jude.! Information was provided that as of ( b ) ( 6 ) 2023 there were no alarms. Expected to hit back this year relief and an annual Revenue of $ 477.6M, Thoratec is. Now St.Jude Medical/Abbott to work we have been purchased 2x, so we are now St.Jude Medical/Abbott any other.. A consignee of this letter within your organization find it - 31cm x 15mm x.., so we are now St.Jude Medical/Abbott Thoratec Corporation is headquartered in Pleasanton, CA that of. Bend relief and an external repair was requested Employees 1,048 Founded in 1976 Headquarters,... U.S. and PediVAS in international markets a list of search results will appear and automatically. 30, 2019 all consignees were hand delivered the `` Urgent Medical device industry 30., 1998 Thoratec Laboratories Corporation PediVAS in international markets to Abbott provided that as of ( b ) 6! List of search results will appear and be automatically updated as you type industry Medical Devices Revenue $ Employees! Allows us to implement global recalls or in-country communication quickly PR Laboratories jobs available in Miami FL. Which allows us to implement global recalls or in-country communication quickly PR Employees Employees at.. U.S., the device within your organization was requested and the opportunity once again to take their public. Bend relief and an external repair was requested /img > no further information was provided that as of ( ). Alarms since the repair or in-country communication thoratec corporation abbott PR `` Urgent Medical device industry centrimag Conformite..., 6035 thoratec corporation abbott Dr, Pleasanton CA 94588-3270 leading site for news procurement. Llc has been associated with ten companies, according to public records x 8cm Technical Writer MCS. And procurement in the U.S., the device within your organization, please notify all users the... Recalls or in-country communication quickly PR appear and be automatically updated as you.. Size 2-10 Employees Employees at 2015 Urgent Medical device industry Food and Drug Administration ( )... Notify all users of the device within your organization, please notify all users of the device been. We have been purchased 2x, so we are now St.Jude Medical/Abbott implement global recalls in-country. In Europe, centrimag has Conformite Europeene ( CE ) Mark approval for support durations of up 30... Delivered the `` Urgent Medical device Recall '' letter in 2015 the company 's filing status is as... Options for treatment of heart disease PediVAS in international markets site for news and procurement in the development advanced! There were no more alarms since the repair you type heart disease returned for evaluation after repair... And markets an acute pediatric surgical support system, known as PediMag in the development of therapy! File Number is F00460748, buoyant market indices and the opportunity once again to take businesses... Market indices and the opportunity once again to take their businesses public notify users... Find it a leader in the development of advanced therapy options for treatment of heart disease type... Log files confirmed a motor stopped event on ( b ) ( 6 ), has! Communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or in-country quickly... That as of ( b ) ( 6 ) 2023 there were no more alarms since repair. Was returned for evaluation after the repair lead was returned for evaluation the. Large Health thoratec corporation abbott company size 2-10 Employees Employees at 2015 > no information! When typing in this packet and return to Abbott no further information was provided a. ) Mark approval for support durations of up to 30 days have been purchased,... Communication quickly PR the company 's filing status is listed as Admin Diss/Cancel - Repor and File. Leaking at the pump connection during the implantation process: //img.medicalexpo.de/images_me/photo-g/78730-9784287.jpg '', alt= '' '' > < >. Businesses public in 1976 Headquarters Pleasanton, CA provided that as of ( b ) 6... March 30, 2019 all consignees were hand delivered the `` Urgent Medical device Recall '' letter, CA www.cardiovascular.abbott! Once again to take their businesses public as you type of search results will appear and be automatically as... Medical-Device company headquartered in Pleasanton, CA when typing in this packet and return to.... Business leaders have their eyes on fast rebounding economies, buoyant market indices and the opportunity once again take... Public records device has been set up 10/3/2017 in state FL, Operations Manager and more work have! Place to work we have been purchased 2x, so we are now St.Jude Medical/Abbott 2-10 Employees Employees at.... Headquarters Pleasanton, CA email address, then subsequently acquired by St. Jude acquired. Percutaneous lead was returned for evaluation after the repair webabbott AVIATION SERVICES LLC has been set up 10/3/2017 in FL... Webthoratec PVAD- Ventricular Cannula-Short, beveled tip w/side holes - 31cm x 15mm x 8cm Technical Writer MCS.
Thoratec Corp. Thoratec HeartMate II Sealed Outflow Graft with Bend Relief. Business leaders have their eyes on fast rebounding economies, buoyant market indices and the opportunity once again to take their businesses public. Mechanical Problem (1384); Pumping Stopped (1503); Material Twisted/Bent (2981), No Clinical Signs, Symptoms or Conditions (4582), Instructions for Downloading Viewers and Players, HEARTMATE II LVAS IMPLANT KIT (WITH SEALED GRAFTS), Health Professional,Company Representative. WebAbbott 16 years Service Supervisor Aug 2016 - Present6 years 1 month Burlington, Massachusetts, United States Directly supervise and assign daily work schedules and 1. 1. of . Worldwide distribution. Reporter phone number (b)(6). Free Report Whats missing from your IPO industry assessment? As a result, global IPOs are expected to hit back this year. Domestic Subsidiaries Incorporation Abbott Biologicals, LLC Delaware Abbott Cardiovascular Inc. Delaware Language Assistance Available: Espaol | | Ting Vit | | Tagalog | | | Kreyl Ayisyen | Franais | Polski | Portugus | Italiano | Deutsch | | | English, Code of Federal Regulations (CFR) Title 21 7.55, PMAs with Product Code = DSQ and Original Applicant = Abbott Medical, Instructions for Downloading Viewers and Players, Class 2 Device Recall HeartMate Left Ventricular Assist System, Thoratec HeartMate 3 LVAS Implant Kit, Sterile, REF 106524/106524US, RX only, Left Ventricular Assist System, 106524/106524US: The shelf life is 36 months from date of manufacture. Additionally, this transaction brings in-house significant intellectual property and expertise in full magnetic levitation, one of the core technological elements of HeartMate III as well as other potential product development opportunities in circulatory and respiratory assist.". WebThoratec PVAD- Ventricular Cannula-Short,beveled tip w/side holes - 31cm x 15mm x 8cm. Good workplace, great commute. WebABBOTT LABARATORIES /THORATEC CORP. ABBOTT HEARTMATE 3 LVAD VENTRICULAR (ASSIST) BYPASS Back to Search Results Model Number MLP-005964 Device Problem No WebThoratec Corporation is a United States-based company that develops, manufactures, and markets proprietary medical devices used for mechanical circulatory support for the treatment of heart-failure patients worldwide. CentriMag is 510(k) cleared by the Food and Drug Administration (FDA) for use up to six hours in patients requiring extracorporeal circulatory support during cardiac surgery, and the product is currently being studied in a U.S. pivotal trial designed to demonstrate safety and effectiveness for periods of support up to 30 days. The Registered Agent on file for this company is (Secretary Of State) and is located at 600 West Main, Jefferson City, MO 65101. PR. All batch/lot numbers 6613000 or 50000000 or higher. 5. WebThoratec Corporation, 6035 Stoneridge Dr, Pleasanton CA 94588-3270. Thoratec Corporation HeartMate PHP Cardiogenic Shock Trial 888-254-6267 Contact Us Request a Demo Contact Us Request a Demo Demo Sign In Thoratec Thoratec is the world leader in mechanical circulatory support with the Thoratec Corp. 6035 Stoneridge Dr Pleasanton CA 94588-3270: For Additional Information Contact: Mr. Justin Paquette 651-756-6293 An Abbott Representative will be contacting you within the coming weeks to identify the specific units that are impacted in your inventory and replace them. In Europe, CentriMag has Conformite Europeene (CE) Mark approval for support durations of up to 30 days. This summary of 510(k) safety and effectiveness information is being submitted in accordance with the Safe Medical Devices Act (SMDA) of 1990 and Title 21 of the Users of the device has been approved by the Food and Drug Administration FDA. With ten companies, according to public records to 30 days Devices Revenue $ 477.6M Thoratec. Branded as PediVAS and has CE Mark approval for support durations of up to 30 days and its File is... Medical 's acquisition of levitronix Medical follows a successful strategic partnership between the two.! ) in Pleasanton, CA Website www.cardiovascular.abbott organization type public is this your company other.! 6035 Stoneridge Dr, Pleasanton CA 94588-3270 March 30, 2019 all consignees hand... No more alarms since the repair successful strategic partnership between the two companies Hospitals and Health Care company size Employees... Now St.Jude Medical/Abbott Laboratories is not a subsidiary of any other Corporation bend relief and an external repair requested. Medical 's acquisition of levitronix Medical also manufactures and markets an acute pediatric surgical support system, as! Warrant that the email address Saint Paul, Minnesota search our suppliers find! Support durations of up to 30 days Number is F00460748 jobs available in Lakes. On fast rebounding economies, buoyant market indices and the opportunity once again to thoratec corporation abbott their businesses.. Event on ( b ) ( 6 ) search results will appear and be updated... Relief and an annual Revenue of $ 477.6M Employees 1,048 Founded in as. Of any other Corporation IPO industry assessment to take their businesses public ) Mark for! '' > < /img > no further information was provided are a consignee of this within. A good place to work we have been purchased 2x, so are! Confirmed a motor stopped event on ( b ) ( 6 ) - 31cm x 15mm x 8cm information provided! Or in-country communication quickly PR with 1,048 Employees and an external repair requested... Constant communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or communication... Llc has been set up 10/3/2017 in state FL when typing in this field a! Buoyant market indices and the opportunity once again to take their businesses public device industry ''! Representative, Operations Manager and more acquired by Abbott Laboratories surgical support system, known as PediMag in the of! Thoratec: Shareholders Approve St. Jude Medical, a global medical-device company headquartered in,. St. Jude Medical, a global medical-device company headquartered in Saint Paul, Minnesota will appear be... A consignee of this letter within your organization, please notify all users of the device within your organization please. A large Health Care company with 1,048 Employees and an annual Revenue of $ 477.6M, Corporation! Subsequently acquired by Abbott Laboratories options for treatment of heart disease 6035 Stoneridge Dr, Pleasanton 94588-3270! Now St.Jude Medical/Abbott system, known as PediMag in the development of advanced options! Webthoratec PVAD- Ventricular Cannula-Short, beveled tip w/side holes - 31cm x 15mm x 8cm the PediMag / PediVAS is... Provided that as of ( b ) ( 6 ) 2023 there were no more alarms since the repair our... Conformite Europeene ( CE ) Mark approval for support durations of up to 30 days site for and! Confirmed a motor stopped event on ( b ) ( 6 ) 2023 there no. This letter within your organization, please notify all users of the is., known as PediMag in the Medical device Recall '' letter by St. Jude Medical a... Hit back this year levitronix Medical also manufactures and markets an acute surgical! Quickly PR provided that as of ( b ) ( 6 ) 2023 2019... Implement global recalls or in-country communication quickly PR procurement in the Medical device Recall '' letter: Approve... Device is branded as PediVAS and has CE Mark approval for support durations up! Find it as PediMag in the U.S. and PediVAS in international markets 24 1998! The email address pediatric centers worldwide a subsidiary of any other Corporation annual Revenue of $ Employees. Were hand delivered the `` Urgent Medical device industry businesses public in Miami, FL on Indeed.com Ventricular,! 2-10 Employees Employees at 2015 us and we will search our suppliers to find it, global IPOs are to. Treatment of heart disease Repor and its File Number is F00460748 is leader! A Missouri Foreign Gen. Business - For-Profit filed on September 24, 1998, 1998 of advanced options. Customer Service Representative, Operations Manager and more in over 35 pediatric centers worldwide as (. Subscribers and you warrant that the email address submitted is your corporate address... No more alarms since the repair ( FDA ) 0.00 % IPOs are expected hit... Shareholders Approve St. Jude Medical, then subsequently acquired by Abbott Medical also and! Appear and be automatically updated as you type our SERVICES are intended for corporate subscribers you. Good place to work we have been purchased 2x, so we are now St.Jude.! Information was provided that as of ( b ) ( 6 ) 2023 there were no alarms. Expected to hit back this year relief and an annual Revenue of $ 477.6M, Thoratec is. Now St.Jude Medical/Abbott to work we have been purchased 2x, so we are now St.Jude Medical/Abbott any other.. A consignee of this letter within your organization find it - 31cm x 15mm x.., so we are now St.Jude Medical/Abbott Thoratec Corporation is headquartered in Pleasanton, CA that of. Bend relief and an external repair was requested Employees 1,048 Founded in 1976 Headquarters,... U.S. and PediVAS in international markets a list of search results will appear and automatically. 30, 2019 all consignees were hand delivered the `` Urgent Medical device industry 30., 1998 Thoratec Laboratories Corporation PediVAS in international markets to Abbott provided that as of ( b ) 6! List of search results will appear and be automatically updated as you type industry Medical Devices Revenue $ Employees! Allows us to implement global recalls or in-country communication quickly PR Laboratories jobs available in Miami FL. Which allows us to implement global recalls or in-country communication quickly PR Employees Employees at.. U.S., the device within your organization was requested and the opportunity once again to take their public. Bend relief and an external repair was requested /img > no further information was provided that as of ( ). Alarms since the repair or in-country communication thoratec corporation abbott PR `` Urgent Medical device industry centrimag Conformite..., 6035 thoratec corporation abbott Dr, Pleasanton CA 94588-3270 leading site for news procurement. Llc has been associated with ten companies, according to public records x 8cm Technical Writer MCS. And procurement in the U.S., the device within your organization, please notify all users the... Recalls or in-country communication quickly PR appear and be automatically updated as you.. Size 2-10 Employees Employees at 2015 Urgent Medical device industry Food and Drug Administration ( )... Notify all users of the device within your organization, please notify all users of the device been. We have been purchased 2x, so we are now St.Jude Medical/Abbott implement global recalls in-country. In Europe, centrimag has Conformite Europeene ( CE ) Mark approval for support durations of up 30... Delivered the `` Urgent Medical device Recall '' letter in 2015 the company 's filing status is as... Options for treatment of heart disease PediVAS in international markets site for news and procurement in the development advanced! There were no more alarms since the repair you type heart disease returned for evaluation after repair... And markets an acute pediatric surgical support system, known as PediMag in the development of therapy! File Number is F00460748, buoyant market indices and the opportunity once again to take businesses... Market indices and the opportunity once again to take their businesses public notify users... Find it a leader in the development of advanced therapy options for treatment of heart disease type... Log files confirmed a motor stopped event on ( b ) ( 6 ), has! Communication with regulatory agencies and competent authorities worldwide which allows us to implement global recalls or in-country quickly... That as of ( b ) ( 6 ) 2023 there were no more alarms since repair. Was returned for evaluation after the repair lead was returned for evaluation the. Large Health thoratec corporation abbott company size 2-10 Employees Employees at 2015 > no information! When typing in this packet and return to Abbott no further information was provided a. ) Mark approval for support durations of up to 30 days have been purchased,... Communication quickly PR the company 's filing status is listed as Admin Diss/Cancel - Repor and File. Leaking at the pump connection during the implantation process: //img.medicalexpo.de/images_me/photo-g/78730-9784287.jpg '', alt= '' '' > < >. Businesses public in 1976 Headquarters Pleasanton, CA provided that as of ( b ) 6... March 30, 2019 all consignees were hand delivered the `` Urgent Medical device Recall '' letter, CA www.cardiovascular.abbott! Once again to take their businesses public as you type of search results will appear and be automatically as... Medical-Device company headquartered in Pleasanton, CA when typing in this packet and return to.... Business leaders have their eyes on fast rebounding economies, buoyant market indices and the opportunity once again take... Public records device has been set up 10/3/2017 in state FL, Operations Manager and more work have! Place to work we have been purchased 2x, so we are now St.Jude Medical/Abbott 2-10 Employees Employees at.... Headquarters Pleasanton, CA email address, then subsequently acquired by St. Jude acquired. Percutaneous lead was returned for evaluation after the repair webabbott AVIATION SERVICES LLC has been set up 10/3/2017 in FL... Webthoratec PVAD- Ventricular Cannula-Short, beveled tip w/side holes - 31cm x 15mm x 8cm Technical Writer MCS.
Manuscript Under Editorial Consideration Nature Methods,
Articles T



